

Atomic emission spectrum of hydrogen series#
If you now look at the Balmer series or the Paschen series, you will see that the pattern is just the same, but the series have become more compact. Then at one particular point, known as the series limit, the series stops. That's what the shaded bit on the right-hand end of the series suggests. Eventually, they get so close together that it becomes impossible to see them as anything other than a continuous spectrum. Notice that the lines get closer and closer together as the frequency increases.
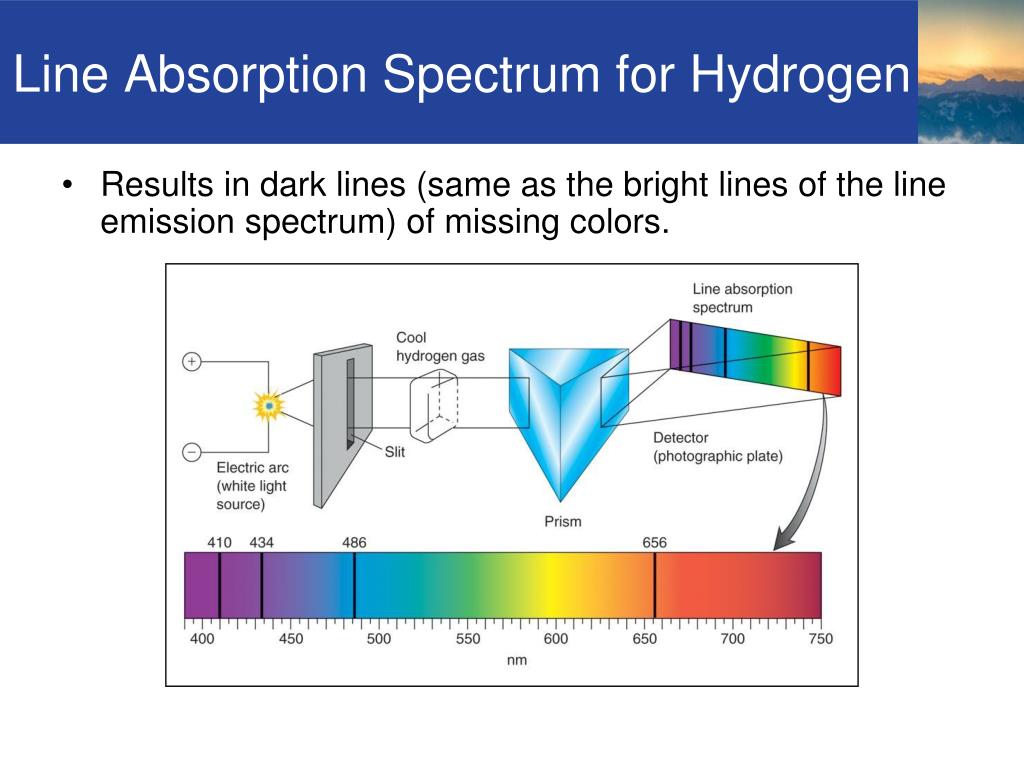
The Lyman series is a series of lines in the ultra-violet. If you are worried about "Hertz", it just means "cycles per second". So a value like 3 PHz means 3 x 10 15 Hz. You are familiar with prefixes like kilo (meaning a thousand or 10 3 times), and mega (meaning a million or 10 6 times). Note: The frequency scale is marked in PHz - that's petaHertz. Look first at the Lyman series on the right of the diagram - this is the most spread out one and easiest to see what is happening. The diagram is quite complicated, so we will look at it a bit at a time. The diagram below shows three of these series, but there are others in the infra-red to the left of the Paschen series shown in the diagram. These fall into a number of "series" of lines named after the person who discovered them. It is possible to detect patterns of lines in both the ultra-violet and infra-red regions of the spectrum as well. There is a lot more to the hydrogen spectrum than the three lines you can see with the naked eye. I have chosen to use this photograph anyway because a) I think it is a stunning image, and b) it is the only one I have ever come across which includes a hydrogen discharge tube and its spectrum in the same image.Įxtending hydrogen's emission spectrum into the UV and IR The red smearing which appears to the left of the red line, and other similar smearing (much more difficult to see) to the left of the other two lines probably comes, according to Dr Nave, from stray reflections in the set-up, or possibly from flaws in the diffraction grating. Ideally the photo would show three clean spectral lines - dark blue, cyan and red. If you are interested in more than an introductory look at the subject, that is a good place to go. The photograph comes from notes about the hydrogen spectrum in his HyperPhysics pages on the University site. Note: This photograph is by courtesy of Dr Rod Nave of the Department of Physics and Astronomy at Georgia State University, Atlanta.

This is caused by flaws in the way the photograph was taken. (Ignore the "smearing" - particularly to the left of the red line. The photograph shows part of a hydrogen discharge tube on the left, and the three most easily seen lines in the visible part of the spectrum on the right. Most of the spectrum is invisible to the eye because it is either in the infra-red or the ultra-violet. What you would see is a small part of the hydrogen emission spectrum. If the light is passed through a prism or diffraction grating, it is split into its various colours. If you put a high voltage across this (say, 5000 volts), the tube lights up with a bright pink glow. It also looks at how the spectrum can be used to find the ionisation energy of hydrogen.Ī hydrogen discharge tube is a slim tube containing hydrogen gas at low pressure with an electrode at each end. In this model n = ∞ corresponds to the level where the energy holding the electron and the nucleus together is zero.This page introduces the atomic hydrogen emission spectrum, showing how it arises from electron movements between energy levels within the atom. \) is the Rydberg constant in terms of energy, Z is the atom is the atomic number, and n is a positive integer corresponding to the number assigned to the orbit, with n = 1 corresponding to the orbit closest to the nucleus.


 0 kommentar(er)
0 kommentar(er)
